| ||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||
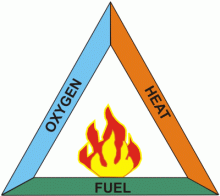
The Basic Elements of FireFire is defined as a rapid, self-sustaining oxidation process accompanied by the evolution of heat and light in varying intensities. It occurs whenever combustible fuel comes into contact with oxygen at an extremely high temperature. Fire is the byproduct of the chemical reaction, known as combustion, in which fuel stored in a combustible fuel is converted into a gas, releasing energy as heat and usually light (a fire’s flames being the visible indication of light). Figure 1. A fire requires fuel to burn, air to supply oxygen, and a heat source to bring the fuel up to ignition temperature. Take away any one of these pillars of the triangle and ultimately the fire will extinguish. Oxygen is almost always abundant; therefore, wildfire management must concentrate on the other two arms of the triangle, i.e. on minimising the possibility of unintentional ignition, and on reducing or eliminating fuel from the potential path of fire. A later section on fire management will detail these options. Fuel The most common fire involves a fuel composed of mostly carbon and hydrogen in an atmosphere with oxygen present. The oxygen combines with the hydrogen to form carbon dioxide. These are the two by-products of complete combustion. Complete combustion occurs rarely due to outside factors. These factors may include but are not limited to, fuel size, fuel arrangements, fuel contaminants, and the availability of oxygen; giving the by-products of incomplete combustion such as smoke, carbon monoxide, and other fire gases. Oxygen Oxygen is the second side of the fire triangle (Fig. 1). It is a naturally occurring element in air. Air is a mixture of approximately 21% oxygen, 78% nitrogen, and the remaining 1% other elements. When a fuel burns it reacts with oxygen from the surrounding air (oxidation), releasing heat and generating combustion products such as gases, smoke and particles. Most fires are burnt in air at 21% oxygen. However, if certain chemical compounds are mixed with, or in close proximity to the fuel, the fire can intensify in this oxygen enriched atmosphere. Other elements, such as chlorine and fluorine, can take the place of oxygen in the combustion reaction. Fluorine is a much stronger oxidiser than oxygen, and a fire in a fluorine atmosphere would burn more rapidly and with more intensity than one occurring in air. Given that urban industrial and residential areas globally are expanding into the margins of forested zones, wildfire managers must be prepared to battle all possible situations and potential oxidisers. Heat A heat source is responsible for the initial ignition of a wildfire, and heat is also needed to maintain the fire and allow it to spread. In addition, heat is constantly emanating from the fire, warming the surrounding air and preheating the fuel in its path. Wildfires can be started both naturally or by humans. Naturally, fires can be started by any natural source of heat, however the only realistic natural ignitions are lightning and lava flows. Unfortunately, the majority of wildfires are the result of human carelessness. Human causes of wildfires include sparks from campfires, discarding lit cigarettes, children playing with matches or fireworks, improperly burning debris, prescribed fires, industrial equipment, and unfortunately the most common, arson. Table 1 illustrates the number of fires burned and the percentage of the total for each cause of wildfires in the USA in 1997. Data is sourced from the National Parks Service (USA) website. Table 1. Number of wildfires and cause of ignition in the USA during 1997
Heat transfer is a critical issue in the study of wildfires. For a fire to grow and spread heat must be transferred from the initial fuel to the surrounding fuel. Heat allows fire to spread by evaporating the moisture from the nearby fuel, thus facilitating spread. The mechanism and the speed of the heat transfer play a great role in the behaviour of wildfires. Figure 2.
For a fuel to catch alight it must be heated to a certain temperature in the presence of oxygen, known as the ignition point. This temperature is unique to every fuel and is the temperature at which combustion continues without any external input of heat; thus the fire becomes self sustaining. This process can be illustrated by the ignition and burning of wood. Wood is made up of long chains of hydrocarbons. As heat is added to wood these long-chained molecules are broken down into shorter and shorter chains, resulting in the shortest chain hydrocarbon molecule, methane, a flammable gas. Several other flammable short chain hydrocarbon molecules will result from this process, including ethane and propane. In the presence of sufficient amounts of heat and oxygen these components will be broken down further into free radicals, with the end result being combustion. Once started fire is easily visualised as a self-sustaining physiochemical reaction, in which vaporised fuel is burned, producing heat, which in turn converts more fuel into vapour, thus continuing the burning cycle. For pyrolysis to take place there must be a way for the heat energy to be transferred from the heat source to the fuel. There are three methods of heat transfer: conduction, convection, and radiation. The modes of heat transfer responsible for the spread of wildfires are principally by convection and radiation, as conduction (the movement of heat through a solid) does not contribute significantly to fire spread because wood and soil are poor heat conductors. Convection, on the other hand, produces most of the fire’s heat. It is the transfer of heat through a circulating medium, such as liquids or gases. An example of this is the heat that flows out of the kettle in a flow of steam. Fires also transfer heat by radiation to the surroundings. Radiation can be defined as the transfer of heat by infra-red electromagnetic radiation, or rays of energy from a heat source. Radiation refers to heat that we can feel without touching, like the heat we feel from the sun even when the air is cold. As these wavelengths of energy travel through space, they eventually come into contact with an object. As the object absorbs the energy, it will become heated, resulting in a rise in temperature. One thing that must be kept in mind when dealing with radiated heat is that it is not affected by wind (Klinoff, 2003). Radiated heat can set fire to objects upwind of the fire, whereas convection-caused fires are much more likely to occur on the downwind side. In the heating process, the moisture in a fuel is first evaporated off (at temperatures above 100ºC); the terpenes, fats, oils and waxes are vaporised (100 – 200ºC); the cellulose is thermally broken down and its breakdown products converted to a vapour (at temperatures above 200ºC); and finally the volatiles ignite to form a visible flame (300 – 400ºC) (Chapman, 1994). Some plants, including particular members of the Eucalyptus and Pinus genera, contain volatile oils and waxes which are highly flammable and release high heat per unit mass. These species are common to southeastern Australia and have been responsible for the spread of many devastating historical wildfires, such as the Ash Wednesday fires of February 16, 1983.
|
||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||